The radiation-curing prepolymer is a low-molecular polymer containing an unsaturated functional group. The coating film component that can be used as a host material for solidification in the field can basically determine the main physical properties of the cured material, and can be used as a printing product. Toughness, adhesion, ink repellency and/or scratch resistance are improved. Oligomer has a large molecular weight, a small volume shrinkage upon curing, and a fast curing speed, but has a large molecular weight and a high viscosity, and is usually used in a small amount to provide desired film forming properties and pigment dispersion.
Non-limiting examples of oligomers are polyester acrylate, polyurethane acrylate and epoxy acrylate, and photoactive hyperbranched resins. Preferably the oligomer is a liquid to maintain the ink with the desired low viscosity, and preferably its functionality is greater than one. If it is not a liquid, it should be soluble in the liquid component of the active substance. Epoxy resins have a very high molecular weight and viscosity, high film shrinkage and limited pigment loading. In view of this, epoxy acrylic oligomers are not suitable for inkjet inks. However, ordinary polyurethane acrylates and vinegar acrylates can provide better overall physical properties, and their viscosities are below the upper limit defined by today's piezo inkjets.
Poly Acrylic Acetate: Polyester acrylate can be obtained when the light base and acrylic acid in the vinegar act. Polyacrylate acrylates have low molecular weights, so the viscosity is also low. The number of functional groups can be from 2 to 10 or more, and a wide range of composition can be used to fine-tune the performance. Its price is very cheap, good wettability and high flexibility. Commonly used as a carrier for grinding pigments. It can sometimes be used as a thinner for UV inks to adjust the viscosity of epoxy acrylates and amine acrylates. It is very strong on the surfaces of non-porous substrates and tantalum sheets, aluminum sheets, and plastic sheets, and is often used for the printing of such articles. Its biggest drawback is its poor chemical resistance. Many alkaline chemicals can erode it. Because of the low molecular weight, the polymerization time is longer. In other words, drying is slower.
Acrylic Polyurethane: When the isocyanate and the fAl groups in the acrylic acid react, ammoniacal acrylate is produced. Number of functional groups 2-6. It is polymerized by UV light and has the characteristics of polyurethane resin. The color film has extremely high brightness, and the performance can be from hard to very soft, good abrasion resistance and scratch resistance, and good adhesion. It can be aliphatic or aromatic isocyanate, and aliphatic isocyanate has very good weathering resistance. The UV ink composed of acrylic polyurethane salt is particularly suitable for printing various plastics and metal foils.
Hyperbranched Acrylic Vinegar: The compact spherical structure of the hyperbranched acrylic vinegar more or less reduces the entanglement of the chain, and the low hyperbranched volume leads to low viscosity while the high concentration of acrylic groups provides attractive physical properties (See Table 2).
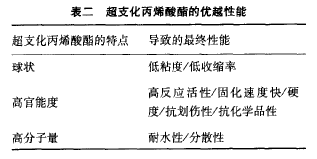
Sartomer already offers commercially available hyperbranched polyalcoholic polyol acrylate vinegar. The degree of conversion of terminal groups to acrylate can easily be varied over a wide range. However, in order to exert the maximum advantage of the hyperbranched structure, the transformation should be as high as possible.
3.3 Photoinitiator
In the UV-curable ink system, a photoinitiator capable of generating free radicals is used. In the ink, the photoinitiator absorbs energy to excite free radicals, thereby initiating the unsaturation in the oligomers and monomers in the ink vehicle. The double bond reacts in a chain reaction to form a three-dimensional solid material, which changes the binder from liquid to solid. Therefore, the photophysical and chemical properties of photoinitiators are very important for photoinitiation and photopolymerization process control. Photoinitiators, which are commonly used for ordinary UV inks, are also suitable for use in UV inkjet inks.
But the choice depends on the choice of colorant and the wavelength of the radiation. The absorption spectrum of the photoinitiator must match the emission band of the irradiation source. In UV inks, because of the strong light absorption and reflection of the pigments, many UV rays that are effective for photoinitiators are shielded. The photoinitiator absorbs light, which leads to a significant decrease in the curing speed of the system and does not even cure. However, most pigments have so-called light-transmitting windows that absorb light relatively weakly in their absorption spectra. Take advantage of these translucent windows. Selecting photoinitiators that match this window is the key to the success of UV inks. In order to achieve a good photocuring of the colored coating, BASF and Ciba Specialty Chemicals have developed novel photoinitiators, TMK, TEPO (BASF) and BAPO (Ciba Specialty Chemicals). The three photoinitiators have good absorption at 400nm, and the photoinitiation efficiency is high; the generated radical absorption moves to shortwave, has a "photobleaching" effect, and is beneficial to the coating curing; the final product is colorless, so it is not Yellowing, ideal for UV-curable inkjet inks. In order to avoid as much as possible the blurring effect of the colorant (especially the pigment), it is preferable to use a mixture of photoinitiators whose peak energy absorption is different in the selected radiation range and which is not sensitive to the wavelength absorbed by the pigment.
(to be continued)
DELIN HAIR COSMETICS , https://www.hairdyecolorfactory.com